Israeli regenerative medicine firmMatricelfhas reached a new milestone in the development of its 3D printed neural implants for paralyzed patients with spinal cord injuries.
The firm has successfully produced its own in-house induced pluripotent stem cells (iPSCs) from human peripheral blood cells, which will be combined with a unique hydrogel to form 3D printed implants which could potentially cure paralysis.
“Our ability to independently produce human iPSCs is a huge achievement of the R&D team both in the scientific and business impacts,” said Asaf Toker, Matricelf’s CEO. “The ability to produce iPSCs without the need to rely on external sub contractures positions the company as a central player in cellular therapy and regenerative medicine industry and will reduce future manufacturing costs.
“We believe that independent manufacturing capacity of iPSCs is a remarkable economic opportunity in billions of USD market per year dealing with spinal cord injury, and in the future for a variety of other medical conditions.”
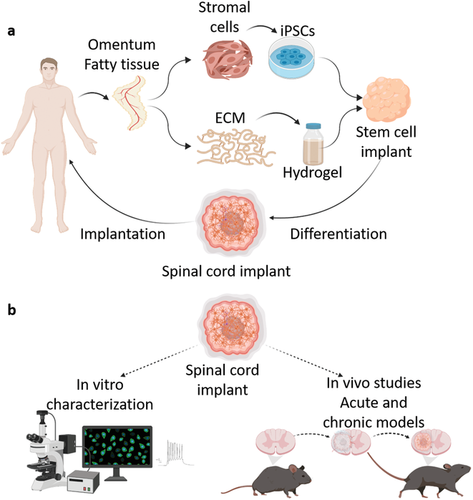
Matricelf’s 3D bioprinting technology
Matricelf成立于2019年,利用了已申请专利的3D生物打印技术,该技术一直在开发特拉维夫大学(TAU) for the last decade in the lab of Professor Tal Dvir, one of the founders of the Matricelf and the firm’s Chief Scientific Officer.
In January, Matricelf revealed it hadsigned an exclusive global licensing agreement与Tau的技术转移公司Ramotto use the technology, which was leveraged to produce what was reportedly theworld’s first 3D printed heartback in 2019.
The bioprinting technology works by simultaneously 3D printing cells and extracellular matrix (ECM) derived from patients to produce living human tissues and organs. Liquid nano-particles stabilize the printed structures and provide high resolution and precision before being extracted after printing is complete.
In February, Matricelf announced its had successfully tested a “first of its kind”3D printed spinal cord tissue implanton paralyzed mice that enabled them to walk again. The firm is currently gearing up to enter into human trials with its 3D printed spinal cord implants in 2024, which they believe could provide a potential cure for paralysis within just a few years.
Fabricating in-house human iPSCs
Matricelf的最新里程碑认为该公司能够首次从人外围血细胞中生产内部IPSC。IPSC能够区分不同的细胞类型,包括神经细胞,并广泛用于治疗目的,例如再生医学,疾病建模和药物发现。
By combining their self-developed iPSCs with a unique thermo-responsive hydrogel, Matricelf will produce 3D printed neural implants that could enable patients with spinal cord injuries to walk again.
The 3D printed implants are designed to enable the regeneration of damaged tissues within and around the spinal cord using a cellular and ECM component originating from individual patients.
Being able to self-manufacture iPSCs is a significant achievement for the company’s pre-clinical R&D program. According to the firm, independently producing iPSCs alongside the development of its hydrogel is a crucial step towards achieving end-to-end independent manufacturing capacity for its neural tissue implants.
“The process of reprogramming mature cells in iPSC, that present the potential to differentiate to any cell type, is a revolutionary and promising technology in the world of cellular therapy and regenerative medicine,” said Tamar Harel Adar, Matricelf’s VP R&D. “Independent manufacturing capabilities of iPSCs is a result of intensive work by the R&D team that is based on acquiring and developing applicable know-how and scientific tools.
“从患者自己的成熟细胞中生产IPSC的能力很重要,因为它使公司能够生产新的组织在各种医疗条件下替代各种人类受损的组织。”

3D生物打印具有很大的潜力enhancing the success rates of spinal surgeriesand treatments, and Matricelf is not the only one making progress in this area.
In terms of research, the last few years have seen the development of3D printed scaffolds containing living cellsthat could help to restore some function to patients with spinal cord injuries. Developed by theUniversity of Minnesota,生物打印的支架由硅胶和干细胞组成,可以在损伤周围的活神经细胞之间形成“桥”。同时,来自University of California San Diegohave successfully 3D printed a two-millimeter spinal cord implant to修复大鼠的脊髓损伤.
Regarding surgical applications, orthopedic implant manufacturer4WEB Medicalhas launched its stand-alone3D printed Anterior Spin Truss Systemdesigned to aid surgeons during spinal procedures, while 3D printed orthopedic device developerOrthofix Medicalhas unveiled itsFORZA Ti PLIF Spacer Systemfor use in posterior lumbar interbody fusion surgeries.
Subscribe to the3D Printing Industry newsletterfor the latest news in additive manufacturing. You can also stay connected by following us onTwitterand liking us onFacebook.
Looking for a career in additive manufacturing? Visit3D Printing Jobsfor a selection of roles in the industry.
Subscribe to ourYouTube channelfor the latest 3D printing video shorts, reviews, and webinar replays.
特色图片显示the stages of Matricelf’s 3D bioprinted spinal cord process. Image via Matricelf.



