Belgian 3D printing software companyMaterialisehas receivedFDAapproval forMimics Enlight, a 3D modeling software for planning cardiovascular surgery.
物质北美物质的副总裁兼总经理布莱恩·克鲁奇菲尔德(Bryan Crutchfield)表示:“我们相信我们的使命是创建一个更健康,更健康的世界[…]我们与合作伙伴医院和医疗设备公司的团队非常紧密地合作,以确定3D计划和印刷可以提高其计划程序的能力的领域。”
“With the FDA clearance of Mimics Enlight, we are expanding the 3D toolkit for cardiologists working to treat patients with complex cardiovascular issues, starting with mitral valve replacement.”
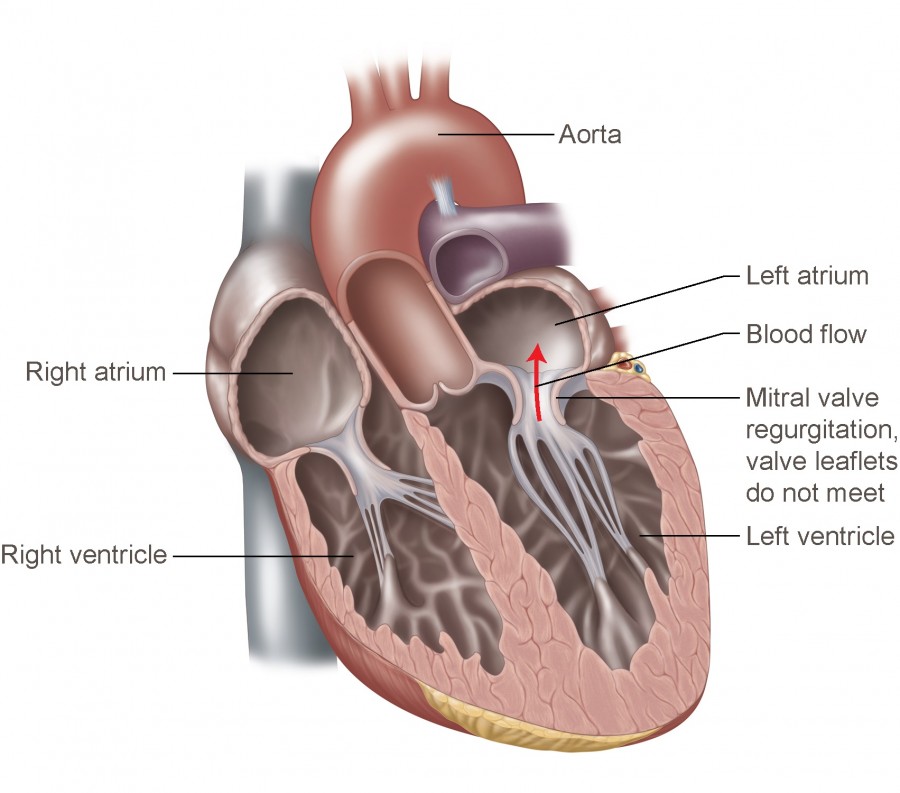
Mitral valve disease
Mimics Enlight was developed in collaboration withHenry Ford Health System, a Detroit-based healthcare organization established by the American industrialist Henry Ford. Dr. Dee Dee Wang, Director of Structural Heart Imaging at theHenry Ford Center for Structural Heart Disease, played an instrumental role in the production of Mimics Enlight.The software is specifically used for planning complex transcatheter mitral valve replacement (TMVR) procedure.
Mitral valve is located between the left atrium and left ventricle of the heart. Among several types of mitral valve diseases, it is reported thatmitral regurgitation is the most common in developed countries, approximately 10% of the over 75 population suffers from the disease.
Although mitral valve repair or replacement is the best treatment for the disease,patients are not referred for surgery as often by their doctors. This is due to the perceived risk of surgery.
Over the years, companies have manufactured devices for less invasive transcatheter mitral valve repair treatments. Such devices include MitraClip by California-basedAbbott Vascular和Carillon byCardiac Dimensionsin Washington.But even though the devices are available for implantation, performing the surgery remains a formidable challenge.
Mimics Enlight was specifically designed to aid the surgical planning of TMVR. The software can design patient-specific 3D models which can be studied for implanting the surgical devices accurately. For effective planning of cardiac surgery, Mimics Enlight also generates reports and workflows.
Materialise and surgical planning
Since therevised guidelines of the FDA, software is considered a medical device. Therefore, 3D modeling software used for planning and performing surgery require FDA approval. As such, Materialise’sMimics Innovation Suite was the first 3D printing softwareto receive FDA approval. Since then Materialise has also validated 3D printers like theUltimaker S5和Stratasys J750和J735for use with Mimics Innovation Suite.
On the FDA approval of Mimics Enlight, Brigitte de Vet-Veithen, vice president of Materialise Medical, said, “Materialise has a wealth of medical technology and experience built throughout two decades of development and implementation of the Mimics Innovation Suite.” de Vet-Veithen added, “That expertise in delivering patient-specific solutions serves as the foundation for Mimics Enlight Mitral’s ability to consistently view and measure each patient’s complex mitral valve anatomy.”
De Vet-Veithen commented on the effectiveness of Mimics Enlight, “Using a 3D model created in Mimics Enlight Mitral improves physicians’ ability to understand and plan procedures before entering the cath lab and gives them the reliable measurements critical to successful implantation of TMVR devices in highly diseased hearts.”
To learn more about how 3D software is changing the medical industry, subscribe to our3D printing newsletter和join us onFacebook和Twitter.
Visit our3D Printing Jobsto start a new career in manufacturing.
Featured image shows planning cardiac surgery with Mimics Enlight. Image via Materialise.

