Medical device manufacturer3D LifePrintshas gained ISO certification for the quality assurance procedures behind its 3D printed surgical guides and anatomical models.
Specifically, the ‘Embedmed’ Quality Management System (QMS) used by the firm to ensure the high standard of its medical products, has been recognized as ISO 13485:2016-compliant. Having met the accreditation’s stringent quality guidelines, 3D LifePrints can now continue offering its patient-specific device range, while weighing-up a potential expansion to its clinical portfolio.
“It has long been our goal to standardize our processes by adherence to this internationally-recognized standard,” said Henry Pinchbeck, CEO of 3D LifePrints. “The formal certification of our QMS opens up considerable market opportunities for the business, and is a clear signal to our customers of the importance we attach to quality assurance and patient care.”
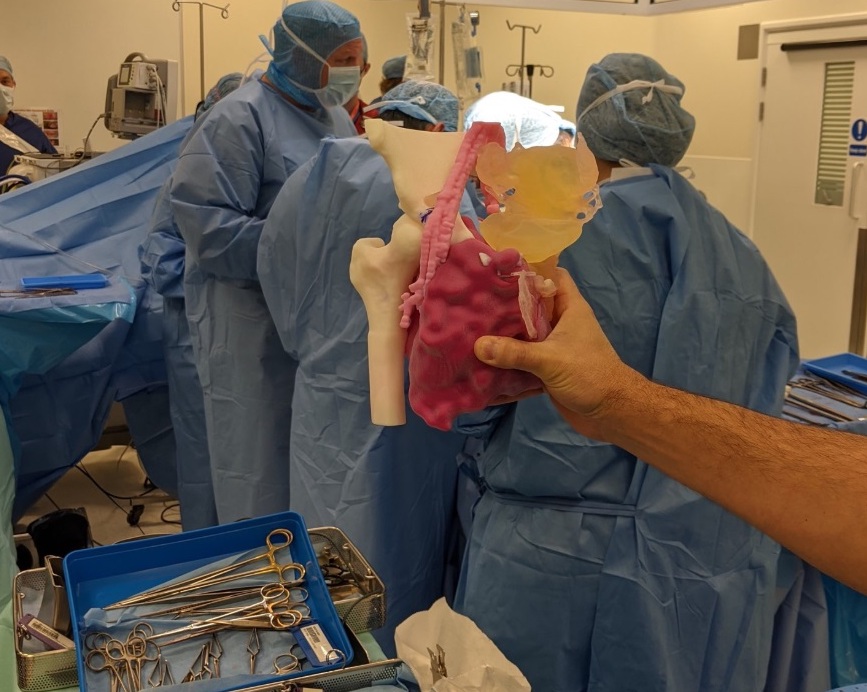
3D LifePrints’ clinical approach
Initially established in 2013 as a humanitarian aid groupproviding prosthetics to African amputees, 3D LifePrints has since expanded, setting up four ‘innovation hubs’ across the UK. At each of these bases, the company offers to 3D print highly-accurate surgical models, which serve to help patients better understand their condition, as well as providing surgeons with a valuable pre-operative training tool.
Thanks to the FDM, SLS, SLA and PolyJet technologies housed at these hubs, 3D LifePrints says that host institutions can “enjoy all of the benefits of an ‘in-house’ service,” without having to spend big on new machines. In the past, using the firm’s install base, surgeons have been able to create cardiac models to reduce the risk of complications, as well as making custom face masks for burn victims.
Through successive funding rounds, the company has also sought to continually expand on its operations over the last three years, securing£500,000 worth of investmentin January 2018. Since then, 3D LifePrints has managed toraise a further £1.2 millionas a means of growing the adoption of its hub-based model, and it’s thought that the firm’s ISO certification will further this strategy moving forwards.

Medical 3D printing QA
3D LifePrints’ newly-announced ISO certification covers the “design and manufacture of sterilisable devices within a controlled environment” at its embedded 3D printing hubs. The award confirms that the firm is in compliance with an internationally-recognized set of guidelines, which are designed to ensure the development, production and shipping of only the highest-quality clinical products.
According to theInternational Standards Organization(ISO) itself,ISO 13485:2016refers to QMS standards which demonstrate that medical devices “consistently meet customer and applicable regulatory requirements.” What’s more, to gain certification, firms must also prove that the products provided by their “associated services,” and “suppliers or external parties” meet these stringent guidelines as well.
Now that the processes behind its Embedmed QMS have been certified, 3D LifePrint says that it’s able to “expand its industry-leading point-of-care model.” At present, the company’s operating model sees it deliver products-as-a-service from hubs embedded in host institutions such as universities, clinical practises and hospitals.
Already, the firm is working with the UK-basedAlder Hey Children’s Hospital,Nuffield Orthopaedic Centre,Wrightington HospitalandLeeds General Infirmary,证明了the quality control that goes into the on-demand devices produced there, it now anticipates further “market opportunities” for its 3D printing business ahead.
Surgical models ‘as-a-service’
Using 3D printing, it’s now possible to produce patient-specific anatomical models with high precision, which offer surgeons a better preoperative insight than that provided by traditional 2D imaging. At the UK-basedQueen Elizabeth Hospital, for instance, surgeons have found that installing aStratasys公司Objet 3D Printer hassaved them 3-4 hours per operation.
Similarly, much like LIfePrint’s model-as-a-service business,Fast RadiusandAxial3Dlaunched a‘DICOM-to-print’ service外科医生和医院在北美最后year. Working in tandem, the companies aim to provide clinicians with access to improved surgical planning via micro-millimeter accurate anatomical models with lead times of less than 48 hours.
Elsewhere, 3D printer manufacturer3D Systemshas iteratively built on the capabilities of itsvirtual surgical planning platform, which was通过美国食品及药物管理局in September 2019. The service effectively allows surgeons to turn CT scans of patients into detailed 3D images, which in turn, can be used as a means of planning highly-complex procedures.
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the3D Printing Industry newsletteror follow us onTwitteror liking our page onFacebook.
For a deeper-dive into additive manufacturing, you can now subscribe to ourYoutubechannel, featuring discussion, de-briefs and shots of 3D printing in-action.
Are you looking for a job in the additive manufacturing industry? Visit3D Printing Jobsfor a selection of roles in the industry.
Featured image shows a surgeon holding a 3D LifePrints 3D printed cardiac model. Photo via 3D LifePrints.



